Scientists from UCLA’s Physical Sciences Division utilize their research to combat COVID-19
Scientists all over the world have displayed how useful and applicable their area of expertise is when facing everyday issues. By applying their area of specialization, they simultaneously advance their field in new ways.
From mathematics to virology, scientists in UCLA’s Division of Physical Sciences have taken the mechanisms they specialize in and applied them to creating tools to combat COVID-19.
“Mathematicians and data scientists can really help make the critical information needed to practitioners and other researchers accessible,” said Deanna Needell, a professor in UCLA’s Department of Mathematics. She uses machine learning – a tool that classifies and groups similar topics – to analyze COVID-related literature.
A specific machine learning method, Nonnegative Matrix Factorization (NMF), allows one to “peek inside” and learn why particular documents are similar. This creates a type of correlation that each document has with existing topics.
“Our hope is that by creating an interface to allow for topic-based search rather than word or term-based search, we would allow researchers and practitioners to more easily and effectively find papers on subjects they are looking for, and find similar papers to those that they might otherwise not be aware of,” said Professor Needell.
NMF and similar machine learning tools can be applied to many areas ranging from identifying COVID with X-ray machines to using data to identify patterns and make automated predictions. With the abundance of information available on COVID, many other topics, ways to interpret, understand, and organize are crucially important.
William Gelbart, a professor in UCLA’s Department of Chemistry and Biochemistry, studies vaccine particles in order to find small-scale solutions and apply them to larger problems such as COVID-19. He is developing a new form of a gene-delivery particle as a general platform for administering RNA vaccines and therapeutic RNA molecules. His lab exploits the ability of purified proteins from particular plant viruses to spontaneously package RNA into protective shells that can be targeted to specific professional antigen-presenting cells or cancer cells.

His lab’s COVID-19 vaccine particle is exceptional because, unlike other mRNA approaches, it involves the use of self-replicating mRNA, and is designed to elicit both a B-cell and a T-cell response. B cells, the cells in charge of making antibodies to neutralize virus particles, and T-cells, the cells responsible for destroying already-infected cells, both have memory that helps prevent and clear viral infection.
Gelbart explains, “With this particle, you have complete control of what is inside because you make it in a test tube from purified mRNA and proteins. If there’s something wrong with it, you know what it is and how to tweak it. You can target particles to where you want them to go.”
In collaboration with Professor Otto Yang in the School of Medicine, he is currently testing their vaccine particles in vitro by incubating them with purified antigen-presenting cells and T-Cells from convalescent COVID-19 patients.
Professor Gelbart’s technology can help treat other diseases such as cancer by generating a killer T cell response. This new technology will not only treat viral diseases like COVID-19, but also serve to prevent them. This same technology can also be used in a variety of cancer immunotherapies involving the eliciting of killer T-cell responses to antigens presented by tumors and cancer cells.
“It’s an exciting time to be a virologist. Vaccine technology and immunology have been permanently transformed by the pandemic,” Gelbart said.
Another UCLA professor in the Department of Chemistry and Biochemistry, David Eisenberg, has focused his research on therapy to mitigate the effects of COVID-19. There has been an intense effort for vaccines, but there is not a lot of emphasis on the importance of therapeutics. Therapeutics are crucially needed to treat COVID patients and be better armed against future pandemics.
Eisenberg’s lab has worked for a decade to discover therapeutics for fibril-related diseases such as Alzheimers. Fibril-related diseases are caused by changes in soluble proteins that make them insoluble. When insoluble, proteins can deposit into organs and damage the tissue. One of the SARS-CoV-2 proteins forms fibrils that function in viral replication, so Eisenberg and his team are searching for inhibitors that slow down or stop the replication of the virus. These inhibitors can later be developed into a drug to treat COVID-19.
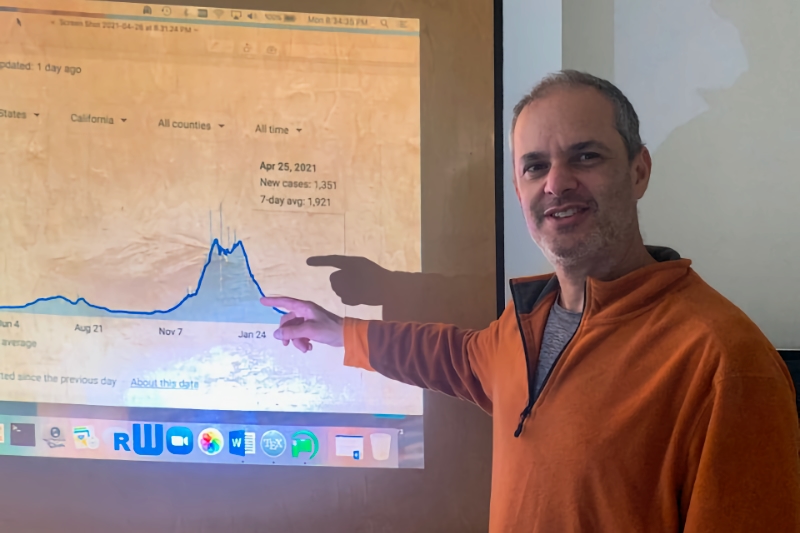
Frederic Schoenberg, a professor in UCLA’s Department of Statistics, has applied a statistical model he utilized when forecasting earthquakes to disease epidemics such as COVID-19. He tailored the Hawkes model – a point process model where each point increases the chance of another point nearby and then each of those can trigger others nearby – to help forecast infected individuals and those who they infect. In the context of COVID-19, each infected person is a “point.”
Usually, Susceptible-Exposed-Infectious-Removed (SEIR) models are used to predict the amount of infected people. However, this model has many parameters that may lead to error.
“Hawkes models almost always outperform the SEIR models, typically with about 20-30% smaller errors in forecasting. If people are really interested in accurate forecasting, I think Hawkes models are the future,” Schoenberg said.
Hawkes models offer a more direct and simple description of the transmission of disease, with fewer parameters to estimate. Hawkes models also rely less on assumptions about what fraction of the population is exposed to the virus but not diagnosed as positive, which can be very tricky to estimate. The model considers each “point” or infected person and their impact on the population, unlike the SEIR model.
Chad Hazlett, an interdisciplinary professor in the Department of Statistics and Department of Political Science, uses the Stability Controlled Quasi-Experiment (SCQE) in his transdisciplinary project to compare “low use” and “high use” periods of different treatments given to COVID-19 patients. Hazlett has developed this approach as a practical tool for learning the effects of new treatments in contexts such as this.
Other methods for making use of data outside of randomized trials typically produce a single result that can be trusted only if investigators can observe and account for all the factors that make the treated and untreated groups different in their potential COVID mortality. The SCQE is one way of characterizing both what’s known from observational evidence and what’s unknown to help clinicians make decisions when there is not enough evidence from randomized trials.

The results shed light on how treatments given outside of randomized trials may work. However, each treatment can be very different for those eligible to receive treatment in real-world circumstances compared to those consenting to randomized trials.
SCQE can prevent cases where confounding bias makes a treatment that is actually harmful look beneficial. Confounding bias refers to differences seen in COVID-19 patients caused by other factors – such as disease severity – not the effect of the treatment itself. SCQE produces suggestions about medicine and its effects and reminds healthcare scientists that they are not positioned to make a conclusion about the benefits of a given treatment.
“Our hope is that this tool enables researchers to provide practicing clinicians and patients with more transparent and safe conclusions about what we are able to determine from observational data. We hope it will aid in making decisions, particularly before clinical trial results are available or for populations not well represented in those trials,” said Hazlett.
The groundbreaking methods developed and applied by these UCLA scientists will contribute not only to the fight against COVID-19, but can be used to help battle other diseases in the future.
Story by Angela Estrada
Tags: News
