A team of scientists at UCLA has uncovered new insights into how cells organize themselves at the molecular level, revealing fundamental rules that govern the formation of mysterious cellular structures and opening new paths for understanding human health and disease.
Assistant Professor Kalli Kappel, who joined UCLA’s Department of Chemistry & Biochemistry on July 1 from the Broad Institute of MIT and Harvard, led the study, which focuses on biomolecular condensates. These structures, which are still not well understood, are dynamic, membrane-less compartments that help organize cellular activity. Although scientists have known about these structures for more than a century, their biological significance has only come into sharp focus over the past 15 years.
Condensates resemble classical organelles such as the nucleus or mitochondria but lack a surrounding membrane. Instead of being enclosed by a physical boundary, they are held together by intrinsic molecular interactions. “Cells contain many specialized compartments, but not all of them are wrapped in membranes,” Kappel said. “That raises a fundamental question. What properties of the molecules themselves cause these structures to form and persist?”
The research, published in Nature Methods, centers on how protein sequences drives condensate formation. While condensates are involved in essential biological processes, they are also frequently disrupted in disease. Understanding how specific sequences give rise to these structures could help explain how genetic mutations interfere with normal cellular function and suggest new ways to target condensates therapeutically.
New hires like Professor Kappel strengthen UCLA Physical Sciences’ already strong leadership in research, enhance educational opportunities for students, and expand the university’s capacity to serve the broader community through scientific discovery.”
– Dean of UCLA Physical Sciences Miguel Garcia-Garibay.
To tackle this challenge, Kappel’s team developed a new experimental approach called CondenSeq, a high-throughput platform that allows researchers to examine thousands of protein sequences simultaneously inside living human cells. Traditional experiments typically study one protein variant at a time, limiting scientists’ ability to uncover general rules. But CondenSeq enables the exploration of protein behavior at an unprecedented scale.
Using the technique, researchers designed large libraries of protein sequences—about 5,000 per experiment—each tagged with a fluorescent marker and a unique DNA barcode. These libraries were introduced into millions of human cells, with each cell expressing a different sequence. The team then used microscopy to observe whether the proteins formed condensates and read out the DNA barcodes directly within the cells to identify which sequence produced which behavior.
“This lets us look across a broad landscape of sequence space,” Kappel explained. “By comparing thousands of sequences at once, we can start to decipher the molecular features that promote or prevent condensate formation.”
The approach combines existing technologies in a way that had not previously been applied to condensates in cells. The resulting dataset provides a detailed map linking protein sequence to cellular organization.
The next phase of the work will push these insights even further. Researchers in the Kappel Lab are now using machine learning to build predictive models capable of determining whether a given protein sequence will form a condensate, as well as designing new sequences with specific properties. These models could ultimately help scientists anticipate how mutations alter cellular organization or engineer proteins that assemble in precise ways.
This research also marks an important milestone for Kappel, for whom UCLA is her first faculty appointment. “Starting a lab is both exhilarating and overwhelming,” she said. “There’s a lot you’re never formally trained to do, but the freedom to pursue the science you care about, and to build a team around that vision, is incredibly rewarding.”
Kappel’s arrival at UCLA Physical Sciences is part of a broader investment in recruiting innovative early-career faculty whose work bridges disciplines and advances foundational science. “New hires like Professor Kappel strengthen UCLA Physical Sciences’ already strong leadership in research, enhance educational opportunities for students, and expand the university’s capacity to serve the broader community through scientific discovery,” said Dean of UCLA Physical Sciences Miguel Garcia-Garibay.
As UCLA researchers continue to unravel the molecular rules that govern cellular organization, the findings could have far-reaching impacts, from improving our understanding of disease to guiding the design of future biological systems.
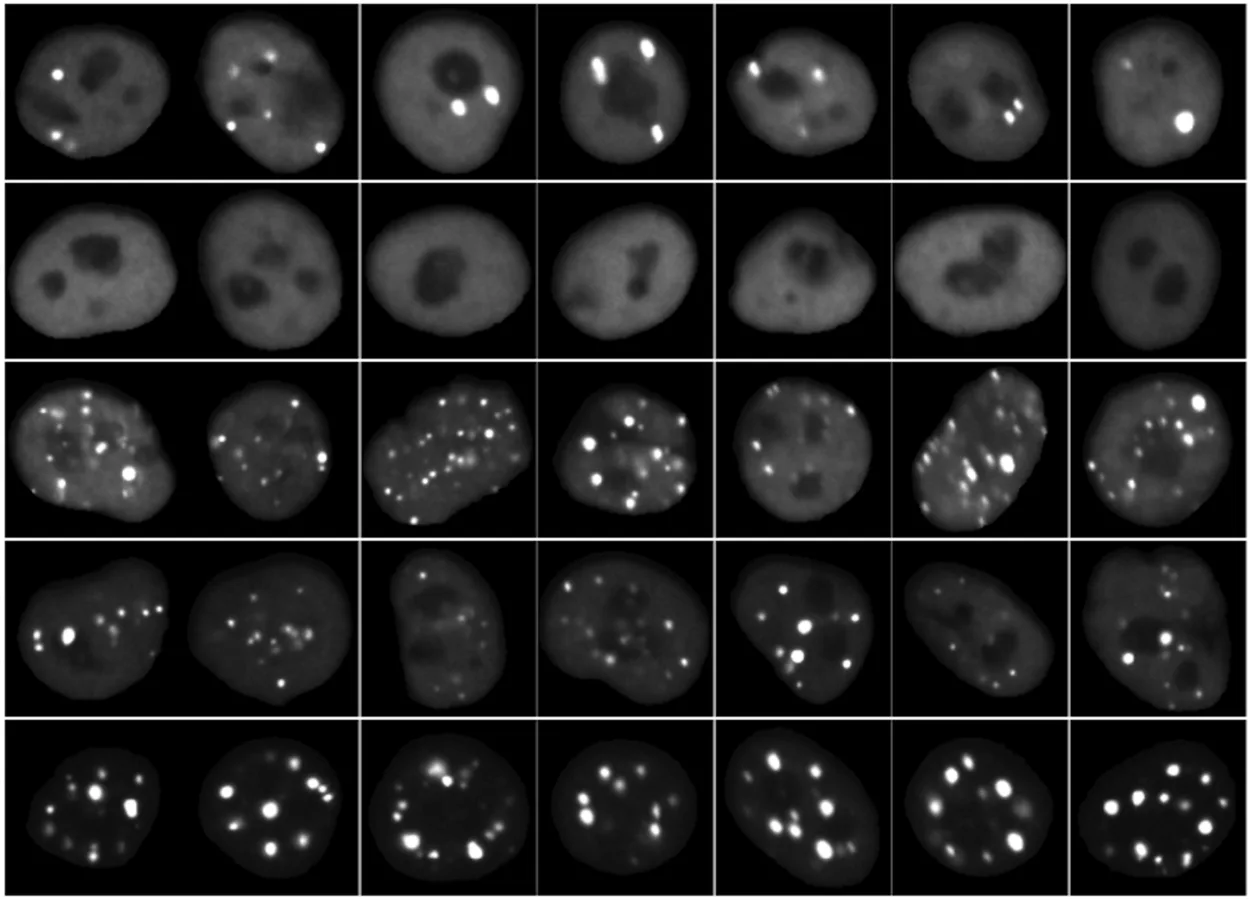 Images of cells expressing different fluorescently tagged protein sequences. Each image is a different cell and each row is contains different cells expressing the same protein sequence (Photo Credit: Kalli Kappel)
Images of cells expressing different fluorescently tagged protein sequences. Each image is a different cell and each row is contains different cells expressing the same protein sequence (Photo Credit: Kalli Kappel)